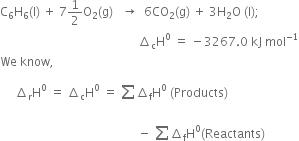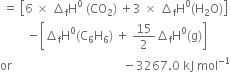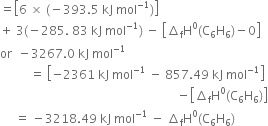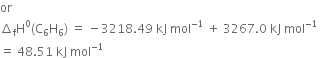The combustion of one mole of benzene takes place at 298K and 1 atm. After combustion, CO2(g) and H2O(l) are produced and 3267.0 kJ of heat is liberated. Calculate the standard enthalpy of formation of ∆fH° benzene, given that standard enthalpy of formation of CO2(g) and H2O(l) are –393.5 kJ mol–1 and –285.83 kJ mol–1respectively.