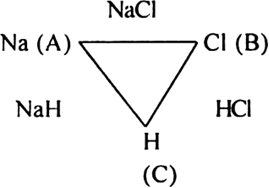
Define the law of reciprocal proportion. Explain with suitable examples.
The law of reciprocal proportion (Richtev 1792) states that if two elements A and B combine separately with another element C, then the ratio of respective weights of A and B which combine with a fixed weight of C is the same or simple multiple of the weights in which A and B combine with each other. For example, sodium and chlorine combine with hydrogen to form compounds NaH and HCl respectively.
In NaH:
23 g sodium combines with 1 g hydrogen.
In HCl:
35·5 g chloride combines with 1 g hydrogen.
The ratio of masses of sodium and chlorine which combine with a fixed mass of hydrogen (1 g) is 23 : 35·5
Now sodium and chlorine also combine to form NaCl.
In NaCl:
23g sodium combines with 35·5 chlorine in the ratio 23 : 35·5
This is the same ratio which combines with one part of hydrogen in NaH and HCl.