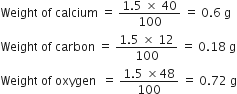If law of constant composition is true, what weights of calcium, carbon and oxygen are present in 1.5 g fo calcium carbonate? Given that a sample of calcium carbonate from another sample contains the following percentage composition:
Ca = 40.0%; C = 12.0%; O= 48.0%
As the law of constant composition is true, therefore, the percentage of the elements in the two samples of calcium carbonate must be same.