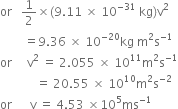The work function for caesium atom is 1.9 eV. Calculate: (a) the threshold wavelength and (b) the threshold frequency of radiation. If the caesium element is irradiated with a wavelength 500 nm, calculate the kinetic energy and velocity of the ejected photoelectron.
Work function ![]()
![]() ...(1)
...(1)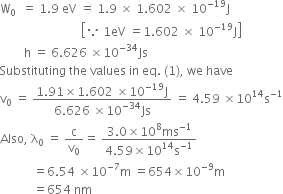
We know, 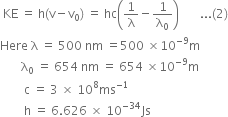
Substituting the values in eq. (2), we have
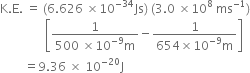
![]()
![]()