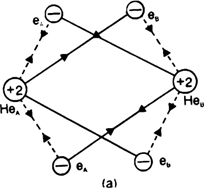
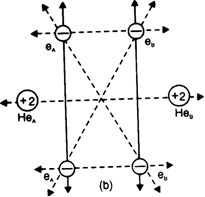
In the light of attractive and repulsive forccs, show that helium molecule is not formed.
Each helium atom has two electrons in its s orbital. When two helium atoms come closer together, the new attractive and repulsive interactions will start operating. There are four attractive interactions between the electrons of Helium.
Dotted lines show new repulsive interactions one and nucleus of the other and vice-versa. There are five new repulsive interactions, one of which is internuclear and the other four are interelectronic repulsions. As the number and magnitude of repulsive forces are more than that of attractive forces, the potential enthalpy of the system increases. Hence a chemical bond is not formed between helium atoms or He2 is not formed.

Thus energetically the formation of helium molecule is not possible because there is an increase in the potential enthalpy of the system when two helium atoms approach each other.
192 Views