Discuss the resonance in CO2 and O3molecule.
Resonance in CO2:
CO2 molecule is a resonance hybrid of the following three contributing structures.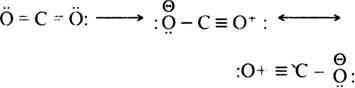
The above different contributing structures of CO
2 molecule differ only in the distribution of electrons and not in the arrangement of atoms. Thus, C to O bond has a single bond, double bond as well as a triple bond character since all the structures contribute towards the hybrid.
Resonance in (Ozone). The structure of ozone molecule is angular. Ozone molecule is the resonance hybrid of the following two structures:
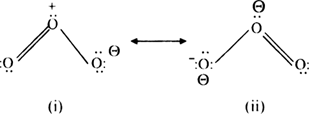
These two contributing structures (i) and (ii) of O
3 molecule differ only in the distribution of electrons.
143 Views