Draw the molecular orbital energy diagram for oxygen molecule (O2) and show that:
(i) It has a double bond
(ii) It has paramagnetic character.
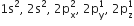 Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:
Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:
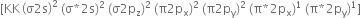

 orbitals, molecular oxygen should be paramagnetic.
orbitals, molecular oxygen should be paramagnetic.