Discuss:
(a) Nitration of an alkane.
(b) Sulphonation of alkane.
(a) Nitration of alkane: The process which involves the replacement of hydrogen atom of alkanes by nitro group (-NO2) is known as nitration of an alkane. It can be carried out in two ways:
(i) Liquid phase nitration; In this method higher alkane is heated with fuming HNO3 at 413 K under pressure.
(ii) Vapour phase nitration: Lower member of alkanes can be nitrated by vapour phase nitration i.e. by heating a gaseous mixture of hydrocarbon and nitric acid vapours at 673-773 K.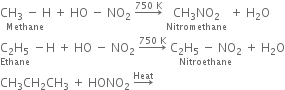
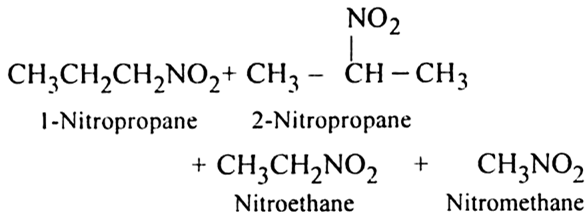
Mechanism of nitration:
The nitration of alkanes proceeds by the free radical mechanism.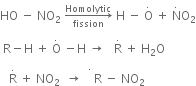
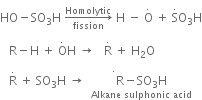
(b) Sulphonation of alkane: The process which involves the replacement of hydrogen atom of alkanes with a sulphonic acid group (-SO3H) is known as sulphonation of alkane.
It is carried out by heating higher alkanes (hexane or higher members) with fuming sulphuric acid.
Mechanism of sulphonation:
The sulphonation of alkanes proceeds by the free radical mechanism.