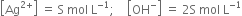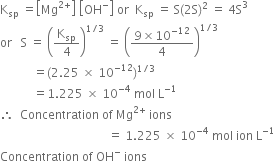Calculate the concentration of Mg2+ ions and OH- ions in a saturated solution of Mg(OH)2, solubility product of Mg(OH)2 is 9×10–12
Let the solubility of Mg(OH)2 be S mole per litre.

i.e. 1 mole of Mg(OH)2 in the solution gives 1 mole of Mg2+ ions and 2 moles of OH- ions.
Hence in the solution,
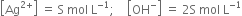
Applying the law of solubility product;
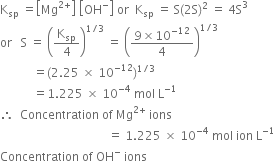
= 2 x 1.225 x 10-4 mol ion L-1
= 2.45 x 10-4 mol ion L-1
108 Views