Discuss the structure of H2O molecule.
In H2O, the oxygen is sp3 hybridised and hence has four sp3 hybrid orbitals. Two of these sp3 hybrid orbitals are half filled and hence overlap with 1s-orbitals of hydrogen to form two O-H bonds. The other two sp3 hybrid orbitals contain a lone pair of electrons each. Due to the presence of four pairs of electrons (two bond pairs and two lone pairs) around the central oxygen atom, the geometry of the molecule is expected to be tetrahedral and ZHOH bond angle should be 109°– 28'. But actually ∠HOH bond angle is 104 . 5°. This is because lone pair-lone-pair repulsions are greater than lone-pair-bond-pair repulsion which in turn are greater than bond pair-bond pair repulsions. As a result ∠HOH bond angle in water is slightly lower than the regular tetrahedron angle (109°–28'). It is 104.5° as shown.
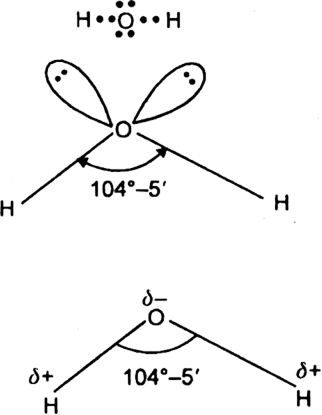
The H2O molecule is of polar nature because of its bent geometry i.e. V-shaped geometry. It has a dipole moment of 1.84D.
197 Views

