
The order of the relative stabilities of different alkyl free radicals are:![]()
There is no scope of any hyperconjugation in methyl free radical. But ethyl free radical is regarded as resonance hybrid of the following four structures: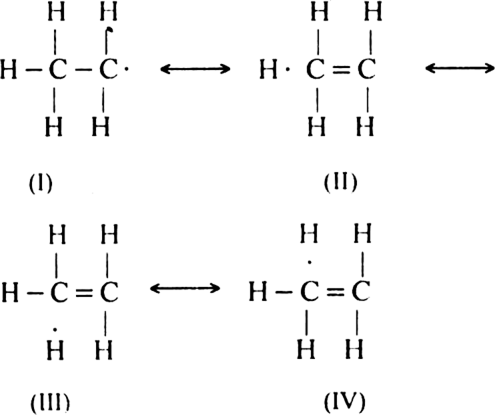
Therefore, ethyl free radical is more stable than the methyl free radical. In the case of isopropyl free radical, there are six contributing structures in addition to the normal structure.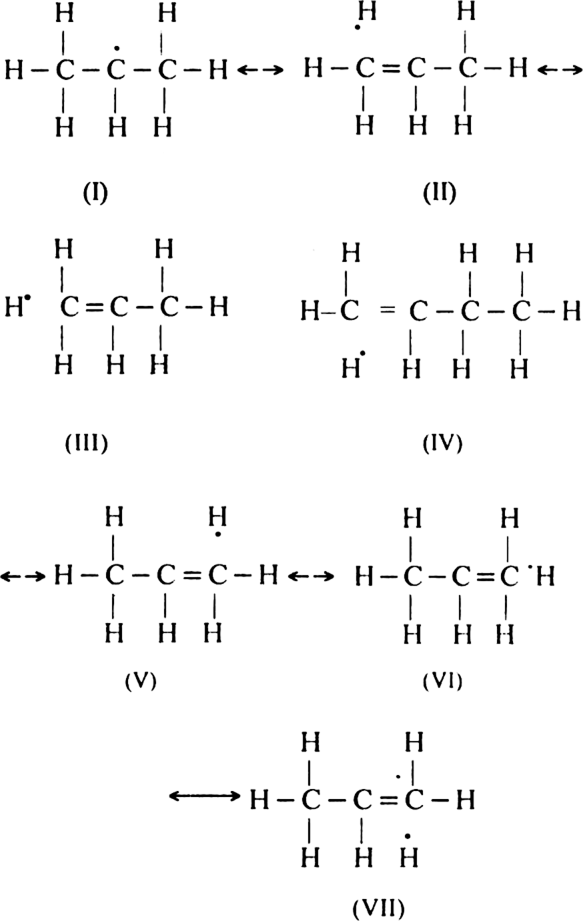
Similarly, nine contributing structures are possible for the tertiary butyl free radical in addition to its normal structure. Therefore, it is still more stable and thus, the order of the relative stabilities of the different alkyl free radicals can be justified.
