
(ii) Structure of IF7: Total number of electrons in the valency shell of the central atom, i.e., I = 7.
No. of electrons provided by the seven F atoms = 7 x 1 = 7.
Total no. of electrons around the cental atoms i.e., = 7 + 7 = 14.
Therefore, total no. of electron pair around the central atom i.e., = 14/2 = 7.
But the total no. of bond pairs = 7.
(Because there are seven I—F bonds)
Therefore, total no. of lone pairs = 7 – 7 = 0 on the basis of VSEPR theory, a molecule with seven bond pairs and no of lone pair must have pentagonal bipyramidal geometry.
Fig. Shapes of IF7 molecule.
Fig. Structure of xenon difluoride.
Structure of XeF2: Xenon difluoride molecule possesses a trigonal bipyramidal structure. The xenon and fluorine atoms lie in a straight line (linear position) while the three lone pairs of xenon occupy the equatorial positions.
Structure of ClO–4: It has tetrahedral shape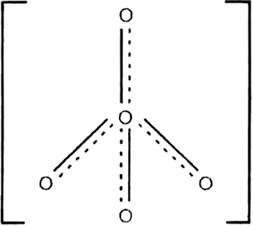
Fig. Shapes of ClO4–.
Structure ICl4–
centre iodine contain 8 electron (7 from iodine and one form negtive charge) In which 4 bond pair and 2 lone pair total 6 pair of electron
thus strycture of ICl4– is square planer 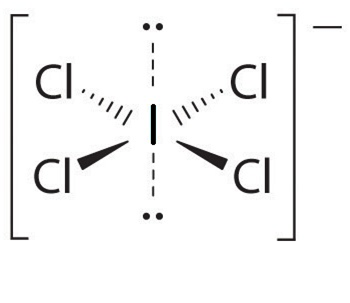
centre iodine contain 8 electron (7 from iodine and one form negtive charge) In which 2 bond pair and 3 lone pair total 5 pair of electron
thus strycture of IBr2– is linear structure IBr2–.
