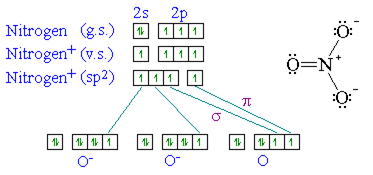
The sp3 hybrid orbitals of the central atom N is displayed. The three sp2 orbital lie in a plane and form a trigonal planar arrangement. Each of the N-O bond is formed by the overlap of a nitrogen sp2 hydride orbital and an oxygen 2p orbital. The NO3- molecule is planar and all ONO angles are 120°.