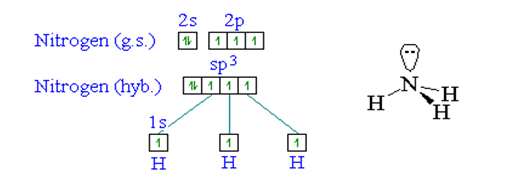
NH3 is Sp3 hybirdisation as there are three bonding pairs of electrons and one non bonding pair. The bond angle in a molecule of ammonia are 107o, a value very close to tetrahedral angle (109o.5').
The central atom has both shared and unshared electron pairs. The shape of ammonia molecule (NH3) is trigonal pyramid.