Cr2+ is strongly reducing in nature. It has a d4 configuration. While acting as a reducing agent, it gets oxidized to Cr3+ (electronic configuration, d3). This d3 configuration can be written as 3t2g configuration, which is a more stable configuration.
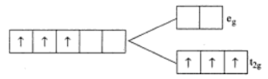
In the case of Mn3+ (d4), it acts as an oxidizing agent and gets reduced to Mn2+ (d5). This has an exactly half-filled d-orbital and has an extra-stability.
