In the given compound, there are two different sets of equivalent b -hydrogen atoms. Thus, dehydrohalogenation of the compound yields two alkenes.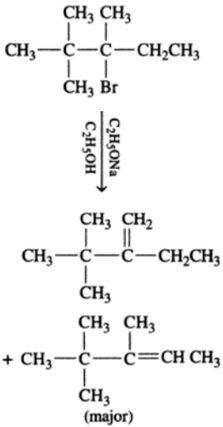
According to saytzeff’s rule in dehydrohalogenation reaction, the alkene having a greater number of alkyl groups attached to the doubly bonded carbon atom is preferably formed. Hence, alkene 3,4,4-trimethylpent-2-ene is the major product in this reaction.
