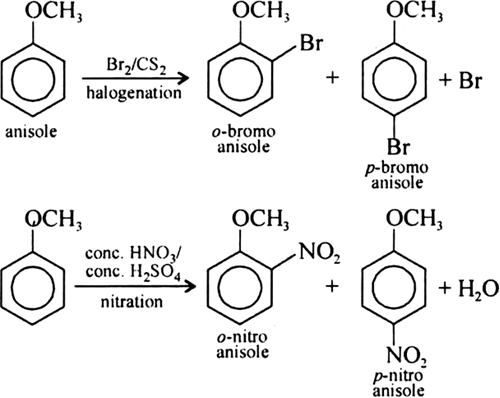
The alkoxy group (—OR) is ortho and para directing group and it activates the aromatic ring in ortho and para positions due to conjugation with pi electrons of the benzene ring. However, ethers are some what less reactive than phenol in these substitution reactions. Activation of benzene ring takes place as shown above.
The following reactions show that alloxy group directs the incoming substituents to ortho and para positions in benzene ring.