Why is benzoic acid a stronger acid than phenol?
Benzoic acid is a stronger acid than phenol because the benzoate ion is stabilised by two equivalent resonance structures in which the negative charge is present at the more electronegative oxygen atom.
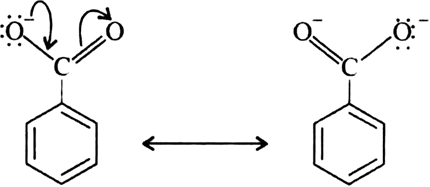
The conjugate base of phenol, a phenoxide ion, has non-equivalent resonance structures in which the negative charge is at the less electronegative carbon atom. Thus, the benzoate ion is more stable than phenoxide ion. Hence, benzoic acid is a stronger acid than phenol.

645 Views


