The density (in g mL–1) of a 3.60 M sulphuric acid solution that is 29% H2SO4 (Molar mass = 98 g mol-) by mass will be
1.64
1.88
1.22
1.22
C.
1.22
Let the density of solution be ‘d’
Molarity of solution given = 3.6
i.e. 1 litre of solution contains 3.6 moles of H2SO4
or 1 litre of solution contains 3.6 × 98 gms of H2SO4
Since, the solution is 29% by mass.
100 gm solution contains 29 gm H2SO4
100/d ml l solution contains 29 gm of H2SO4.
1000 ml solution contains 3.6 × 98 gm of H2SO4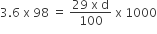
d = 1.22
