(i) Bleaching powder is a yellowish powder with smell of chlorine.
(ii) When in atmosphere, it absorbs moisture but is not deliquescent.
(iii) It is soluble in water but a small insoluble portion is left behind which is the lime present in it.
(iv) Action of carbon dioxide: Upon reaction with carbon dioxide (air), it deteriorates giving off chlorine and calcium carbonate,
CaOCl2(s) + CO2(g) → CaCO3(s) + Cl2(g).
(v) Action of dilute acids: With dilute acids, it liberates the whole of its chlorine,
CaOCl2(s) + H2SO4(ag) → CaSO4(s) + H2O (l) + CI2(g)
CaOCl2(s) + 2HCl(ag) → CaCl2(s) + H2O(l) + Cl2(g).
The chlorine so obtained is known as available chlorine. The value of bleaching powder
in the market is proportional to the quantity of available chlorine liberated by excess of dilute acids. Higher the amount of chlorine liberated, higher is its price.
The bleaching powder in the presence of insufficient dilute acids acts as a bleaching agent due to the liberation of nascent oxygen. Hypochlorous acid is first formed which decomposes to give hydrochloric acid and nascent oxygen. Thus even with a small quantity of the acid, it acts as an oxidising agent and bleaching agent.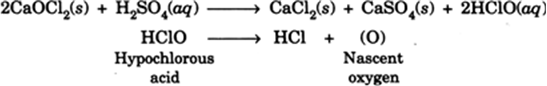
(vi) Effect on long standing: Upon long standing, the following reaction takes place.
6CaOCl2 → 5CaCl2 + Ca(C1O3)2
Thus, the quantity of available chlorine decreases.
