Explain the reaction of dilute acids with metals and metal oxides.
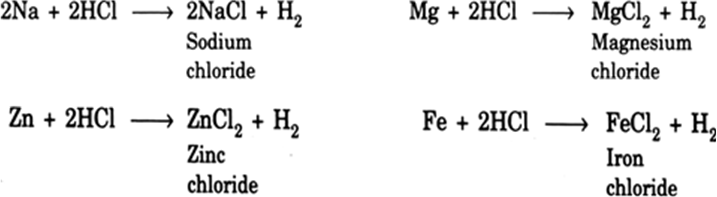
Metals and Acids: Many metals react with dilute acids to give metal salts and hydrogen. The rate of effervescence of hydrogen depends on the reactivity of the metal. Higher rate of effervescence means higher reactivity of the given metal. The reaction of metals with hydrochloric acid and dilute sulphuric acid are similar. With dilute hydrochloric acid (HCl), they give metal chlorides and hydrogen; with dilute sulphuric acid (H2SO4), they give metal sulphates and hydrogen. Nitric acid is an oxidising agent, so it reacts differently.
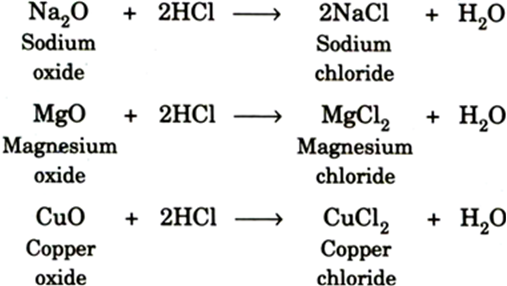
Metal Oxides and Acids: Metal oxides dissolve in dilute acids to give salt and water.
662 Views


