How many aluminium atoms would combine with oxygen atoms to form aluminium oxide? Explain.
An aluminium atom (2, 8, 3) loses 3 electrons to achieve noble gas configuration of neon gas and forms an aluminium cation (2, 8) having three positive charges. An oxygen atom (2, 6) attains noble gas configuration of neon gas after accepting two electrons and forms an oxide anion with two negative charges. As the final compound is electrically neutral, the positive charges in aluminium oxide compound must be balanced by equal number of negative charges. This is possible when 2 aluminium cations (6+ charge) combine with 3 oxide anions (6– charge).
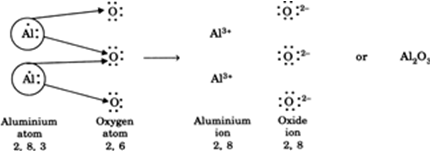
Thus, the ratio between aluminium cations and oxygen anions to form aluminium oxide is 2 : 3. This is indicated by the chemical formula 2Al3+ ? 302– or Al2O3.
281 Views

