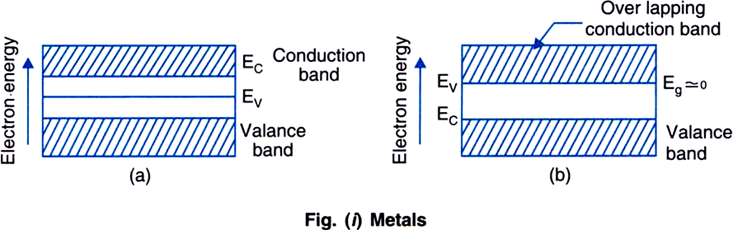
figure (i) b). In both the situations, it can be considered that the metal has a single energy band which is partly filled and partly empty.
Many electrons from below the Fermi level, by acquiring a little more energy from any source, can shift to the higher energy levels above the Fermi level in the conduction band and behave as free electrons. In this situation, large number of electrons are available for electrical conduction, hence the resistance of such a material is low or the conductivity is high. Even if a small electric field is applied across the metal, these free electrons start moving in a direction opposite to the direction of electric field. Due to it, a current begins to flow through it and hence metal behaves as a conductor.
Insulators: The energy bad diagram of insulator is shown in figure (ii). Here, the valence band is completely filled, the conduction band is empty and energy gap is quite large (Eg> 3 eV).
For example, in case of diamond, the energy gap is of 6 eV. Since, the valence band is completely filled as per Pauli's exclusion principle, therefore the electrons are not free. Again due to large energy gap, no electron is able to go from the valence band to the conduction band even if electric field is applied. Hence, electrical conduction in these materials is impossible and they behave as insulators.
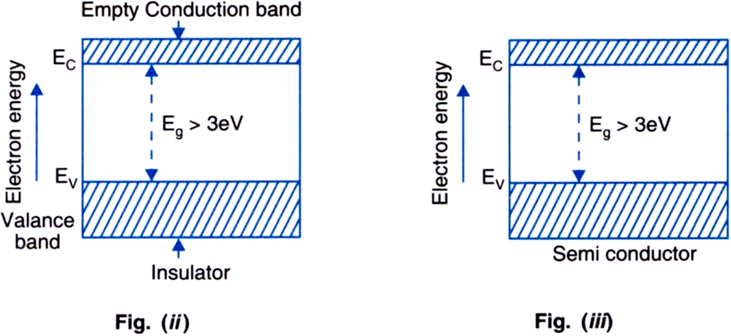
Semiconductors: The energy band diagram of a semiconductor is shown in figure (iii). Here also, the valence band is totally filled and the conduction band is empty but the energy gap between conduction band and valence band is quite small. It is less than 3 eV. For example, the energy gap for germanium is of 0.72 eV and for silicon it is of 1.1 eV. At zero kelvin temperature, electrons are: not able to cross even this small energy gap and hence the conduction band remains totally empty. Therefore, the semiconductor at zero kelvin behaves as insulator. However, at room temperature, some electrons in the valence band acquire thermal energy greater than energy band gap less than 3 eV and jump over to the conduction band where they are free to move under the influence of even a small electric field. As a result of it, the semiconductor acquires small conductivity at room temperature. The resistance of semiconductor would not be as high as that of insulator.
