(a) Write the products formed when CH3CHO reacts with the following reagents:
(i) HCN
(ii) H2N−OH
(iii) CH3CHO in the presence of dilute NaOH
(b) Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Benzoic acid and Phenol
(ii) Propanal and Propanone
OR
(a) Account for the following:
(i) Cl−CH2COOH is a stronger acid than CH3COOH.
(ii) Carboxylic acids do not give reactions of the carbonyl group.
(b) Write the chemical equations to illustrate the following name reactions:
(i) Rosenmund reduction
(ii) Cannizzaro's reaction
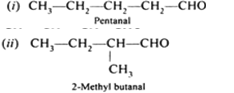
(c) Out of CH3CH2−CO−CH2−CH3 and CH3CH2−CH2−CO−CH3, which gives iodoform test?
(i) Acetaldehyde (CH3CHO) reacts with hydrogen cyanide HCN to give 2-Hydroxypropapanenitrile as product.

(ii) Acetaldehyde (CH3CHO) reacts with Hydroxylamine (NH2OH) to give acetaldoxime as a product.

(iii) The reaction of acetaldehyde with acetaldehyde in the presence of dilute NaOH, this is the kind of Aldol reaction by which obtained 3-hydroxybutanal as a product. Further, proceed reaction when using heat in the reaction, its gives aldol condensation product which is But-2-enal.

(b) Chemical tests to distinguish the following compounds:
(i) Benzoic acid and Phenol : Benzoic acid and phenol can be distinguished by FeCl3 tests. Both reacts with FeCl3 to give different colours. Phenol reacts with FeCl3 to give violet coloured precipitate while benzoic acid gives buff coloured precipitate.
3C6H5OH +FeCl3 ---> (C6H5O)3Fe + 3HCl
phenol violet colour
3C6H5COOH +FeCl3 ---> (C6H5COO)3Fe +3HCl
Benzoic acid buff colour
(ii) Propanal and Propanone : These two are distinguished by the iodoform test.propanal does not give iodoform test when it reacts with I2 in the presence of NaOH while propanone give iodoform test when reacts with I2 in the presence of NaOH.
CH3COCH3 +3NaOI --> CHI3 + CH3COONa +2NaOH
Propane Yellow ppt
CH3CH2OH +NaOI ---> No ppt of CH3I formed
Or
a) (i) Cl-CH2COOH is a stronger acid than CH3COOH :
Substitution of electron withdrawing group on carboxylic acid affect the acidity of the carboxylic acid. Chlorine is a electron withdrawing group and its increase the acidity of carboxylic acids by stabilising the conjugate base due to delocalisation of the negative charge by resonance effects.
Chloroacetic acid ( Cl-CH2COOH) pKa value is equal to 2.7, while pKa value of acetic acid (CH3COOH) is equal to 4.7.
(ii) In carboxylic acid presence of lone pairs of electrons on oxygen which are involves in resonance due to this the electrophilic character of carbon in carboxylic acid decreases. So due to such reason carboxylic acid does not show the characteristic reaction of the carbonyl group.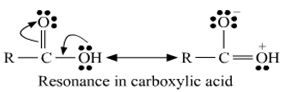
b)
i)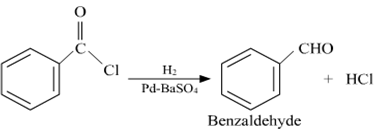
ii)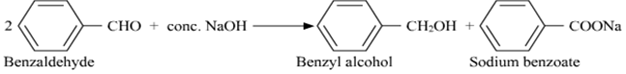
C )
Pentan-2-one (CH3-CH2-CH2-CO-CH3) give yellow precipitate of CHI3 with NaOI, that means it gives iodoform test.
CH3-CH2-CH2-CO-CH3 + 3NaOI --> CHI3 + CH3CH2COONa + 2NaOH
Yellow
ppt.
Pentan-3-one (CH3-CH2-CO-CH2-CH3) does not give yellow precipitate with CHI3 with NaOI, so Pentan-3-one does not give iodoform test.
An organic compound with molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollen's reagent and undergoes Cannizaro reaction. On vigorous oxidation, it gives a dicarboxylic acid which is used in the preparation of terylene. Identify the organic compound.
The positive Brady's test (i.e., reaction with 2, 4-dinitrophenylhydrazine) indicates that the organic compound is carbonyl compound and Tollen's and Cannizaro reactions indicate that it is an aldehyde without -hydrogen atom. As the end product is terephthalic acid, the compound must be p-ethyl benzaldehyde.

Deep blue CuSO4.5H2O is converted to a bluish-white salt at 100oC. At 250oC and 750oC it is then transformed to white powder and black material respectively. Identify the salts.
The Deep blue CuSO4.5H2O is blue vitriol. In its structure, one molecule of water is hydrogen-bonded from 4 sides and thus is released with more difficulty than the rest four water molecules.



(a) Illustrate the following name reactions:
(i) Cannizzaro’s reaction
(ii) Clemmensen reduction
(b) How would you obtain the following?
(i) But-2-enal from ethanal
(ii) Butanoic acid from butanol
(iii) Benzoic acid from ethylbenzene
OR
(a) Given chemical tests to distinguish between the following:
(i) Benzoic acid and ethyl benzoate
(ii) Benzaldehyde and acetophenone
(b) Complete each synthesis by giving missing reagents or products in the following:
(i) Cannizaro reaction
In this reaction, the aldehydes which do not have a a-hydrogen atom, undergo self -oxidation and reduction (disproportionation) reaction on treatment with a concentrated alkali.
Example:
(ii) Clemmensen reduction
In this reaction, the carbonyl compounds are reduced in presence of zinc amalgam to give the corresponding alkane.
![]()
(b)
(i) But-2-enal from ethanal

(ii) Butanoic acid from butanol

(iii) Benzoic acid from ethylbenzene

Or
(a)
(i) Benzoic acid and Ethyl benzoate can be distinguished by sodium bicarbonate test.
Sodium bicarbonate test:
Acids react with NaHCO3 to produce brisk effervescence due to the evolution of CO2gas.Benzoic acid being an acid responds to this test, but ethyl benzoate does not.
![]()
(ii) Benzaldehyde (C6H5CHO) and acetophenone (C6H5COCH3) can be distinguished by iodoform test.
Acetophenone, being a methyl ketone on treatment with I2/NaOH undergoes iodoform reaction to give a yellow ppt. of iodoform. On the other hand, benzaldehyde does not give this test.
b)
