Give a brief account of Kolbe's electrolysis.
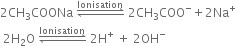
On passing electricity, the ions will move towards the respective electrodes.
At anode: The electron releasing tendency of CH3COO- ions is more and these are discharged in preference to OH- ions.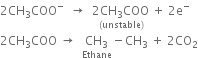
At cathode: The electron accepting tendency of H+ ions is more and these are discharged in preference to Na+ ions which remain in solution.

Limitations:
(i) Only alkanes with an even number of carbon atoms can be formed.
(ii) Methane cannot be prepared by this method.
Discuss:
(a) Nitration of an alkane.
(b) Sulphonation of alkane.
(a) Nitration of alkane: The process which involves the replacement of hydrogen atom of alkanes by nitro group (-NO2) is known as nitration of an alkane. It can be carried out in two ways:
(i) Liquid phase nitration; In this method higher alkane is heated with fuming HNO3 at 413 K under pressure.
(ii) Vapour phase nitration: Lower member of alkanes can be nitrated by vapour phase nitration i.e. by heating a gaseous mixture of hydrocarbon and nitric acid vapours at 673-773 K.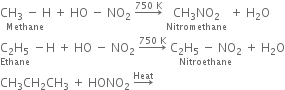
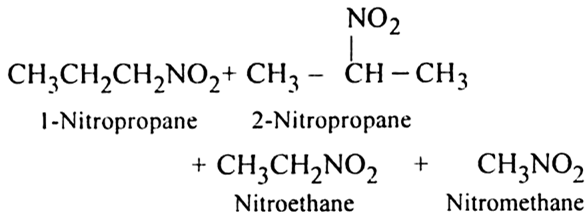
Mechanism of nitration:
The nitration of alkanes proceeds by the free radical mechanism.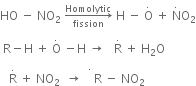
(b) Sulphonation of alkane: The process which involves the replacement of hydrogen atom of alkanes with a sulphonic acid group (-SO3H) is known as sulphonation of alkane.
It is carried out by heating higher alkanes (hexane or higher members) with fuming sulphuric acid.
Mechanism of sulphonation:
The sulphonation of alkanes proceeds by the free radical mechanism.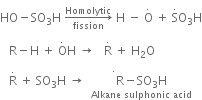
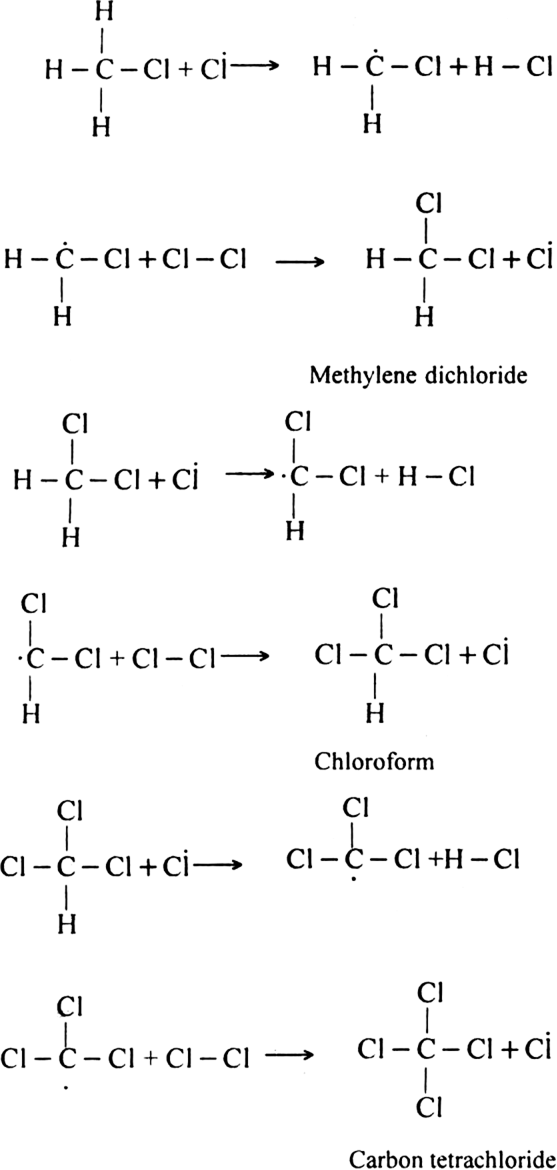
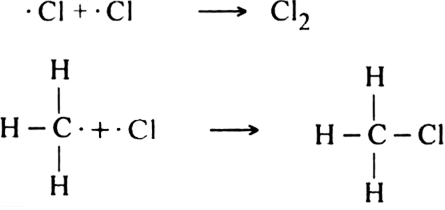
Describe substitution reaction with special reference to halogenation of alkane.
Substitution reaction: A substitution reaction is that which involves the direct replacement of hydrogen atoms of some other groups of a molecule by suitable atoms or groups without changing the structure of remaining part of the molecule. The product obtained is known as substitution product and new atom or group which enters the molecule is known as a substituent.
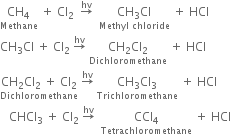
Halogenation: It involves the replacement of one or more hydrogen atom(s) of alkane by the corresponding number of a halogen atom(s).
(i) Chlorination: Chlorination of alkanes is carried out by treating alkane with chlorine in the presence of ultraviolet light or at 523-673 K temperature.
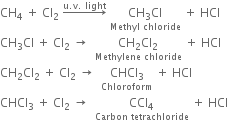
(ii) Bromination: Bromination takes place similarly but not so rapidly.
(iii) Iodination: Iodination is extremely slow and reversible due to the reducing nature of HI.
Thus, iodination is carried out in the presence of some oxidising agent like iodic acid (HIO3) of nitric acid (HNO3) which converts HI to iodine and pushes the reaction in the forward direction.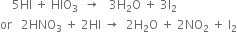
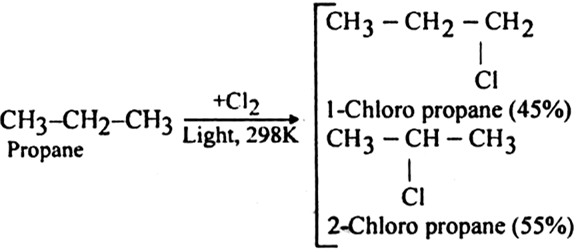
Halogenation of higher alkanes (ethane, propane etc), yields a mixture of all possible isomerism products. 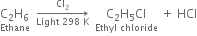

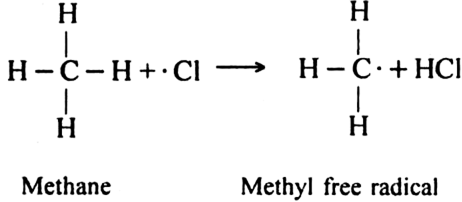
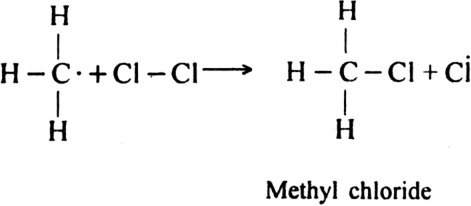
Assign reasons for the following:
(i) Boiling points of n-alkanes increase regularly with the increase in the number of carbon atoms.
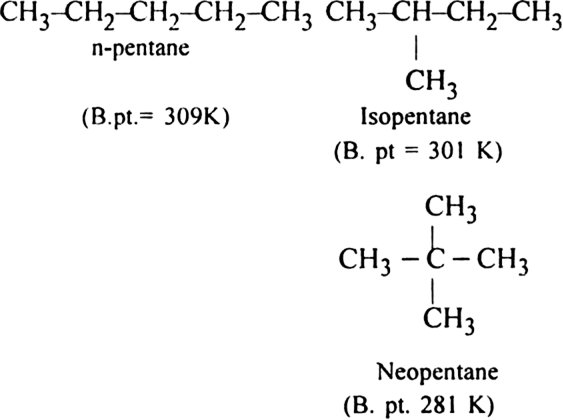
(ii) Branched-chain alkanes have lesser boiling points than the straight chain alkanes.
(i) Alkanes are non-polar molecules and hence are held together by weak Vander Waal’s forces of attraction amongst their molecules. These forces act on the surface of molecules and their magnitude increases with the increase in surface area of the molecules. Thus, with the increase in the number of carbon atoms, the magnitude of Vander Waals forces increases and with that boiling points also increase.
(ii) This is because the branching of the chain makes the molecule more compact and bring the various atoms closer. As a result, the molecular size decreases. This decreases the surface area and hence the magnitude of Vander Waal’s forces (inter-particle forces) and lead to the decrease in boiling point. The boiling points of isomeric alkanes are given below: