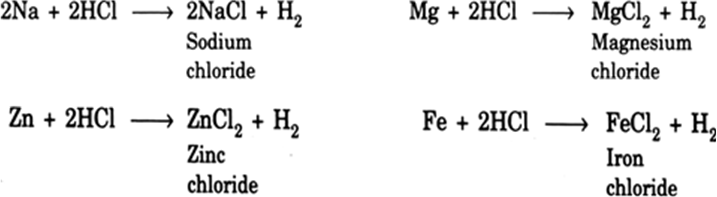
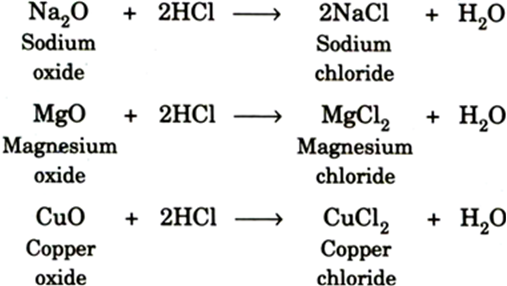
Complete and balance the following equations:
(i) H2CO3 + NaOH →
(ii) CH3COOH + NH4OH →
(iii) HNO3 + KOH →
(iv) H2SO4 + NaOH →
(a) What are these reactions called?
(b) Name the salt formed in each case.

(a) All the above are Neutralization Reactions.
Name of salts in reactions:
(b) (i) Sodium carbonate, (ii) Ammonium acetate,
(iii) Potassium nitrate, (iv) Sodium sulphate.
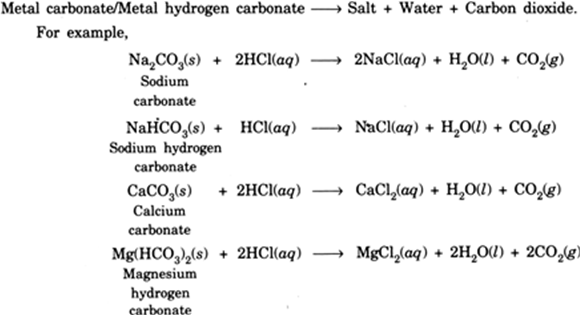
Chemical Reactions:
Marble is calcium carbonate. It reacts with HCl to give CO2
CaCO3 + 2HCl CaCl2 + CO2 + H2O
Marble (A)
CO2 when passed through lime water [Ca(OH)2] gives a white precipitate of CaCO3.
Ca(OH)2 + CO2(g) CaCO3(s) + H2O
White ppt.
(B)
On passing excess CO2, CaCO3 dissolves forming soluble Ca(HCO3)2.
CaCO3 + CO2 Ca(HCO3)2 (aq)
Calcium
hydrogen
carbonate
(C)
A — Carbon dioxide
B — Calcium carbonate
C — Calcium hydrogen carbonate
