Account for the following:
(i) Why does real crystal have more entropy than an ideal crystal?
(ii) Why the entropy of a pure substance is taken as zero at absolute zero?
(iii) Why the entropy of the universe is continuously increasing?
(i) An ideal crystal has a perfect order of its constituent particles while a real crystal has less order because of some defects. Therefore, a real crystal has more entropy than an ideal crystal.
(ii) Because at 0K, there is a complete order in all the crystals and no randomness, therefore, entropy is taken as zero.
(iii) Because, in the universe, almost all processes are spontaneous and for all spontaneous processes, there is an increase in entropy.
What are important consequences of lattice enthalpies?
Important consequences of lattice enthalpies:
(i) The greater the lattice enthalpy, more stable is the ionic compound.
(ii) The lattice enthalpy is greater, for ions of higher charge, and smaller radii.
(iii) The lattice enthalpies effect the solubilities of ionic compounds.
Predict the change in entropy for the system in which the following physical changes occur:
(i) Dissolving cream in a cup of coffee.
(ii) The beating of an egg for an omellete.
(iii) The formation of a rain drop in a cloud.
(iv) The crystallization of a metal alloy from the molten state.
Changes in entropy of the given system:
(i) Increases
(ii) Increases
(iii) Decreases
(iv) Decreases
Calculate the lattice enthalpy of MgBr2 from the given data. The enthalpy of formation of MgBr2 according to the reaction

Using Born-Haber cycle of MgBr2,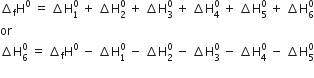
Substituting the values, we have,
= -524 - 148 - 2187 - 31 - 193 - 2 (-331)
= -2421 kJ mol-1
Hence lattice enthalpy = 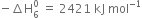
Which of the following reaction will have a greater change in entropy? Explain.![<pre>uncaught exception: <b>Http Error #404</b><br /><br />in file: /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php line 61<br />#0 [internal function]: com_wiris_plugin_impl_HttpImpl_0(Object(com_wiris_plugin_impl_HttpImpl), NULL, 'http://www.wiri...', 'Http Error #404')
#1 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('com_wiris_plugi...', Array)
#2 [internal function]: _hx_lambda->execute('Http Error #404')
#3 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(532): call_user_func_array(Array, Array)
#4 [internal function]: haxe_Http_5(true, Object(com_wiris_plugin_impl_HttpImpl), Object(com_wiris_plugin_impl_HttpImpl), Array, Object(haxe_io_BytesOutput), true, 'Http Error #404')
#5 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/php/Boot.class.php(769): call_user_func_array('haxe_Http_5', Array)
#6 [internal function]: _hx_lambda->execute('Http Error #404')
#7 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(27): call_user_func_array(Array, Array)
#8 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(444): com_wiris_plugin_impl_HttpImpl->onError('Http Error #404')
#9 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/haxe/Http.class.php(458): haxe_Http->customRequest(true, Object(haxe_io_BytesOutput), NULL, NULL)
#10 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/HttpImpl.class.php(40): haxe_Http->request(true)
#11 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/lib/com/wiris/plugin/impl/TextServiceImpl.class.php(80): com_wiris_plugin_impl_HttpImpl->request(true)
#12 /home/config_admin/public/felixventures.in/public/application/css/plugins/tiny_mce_wiris/integration/service.php(19): com_wiris_plugin_impl_TextServiceImpl->service('mathml2accessib...', Array)
#13 {main}</pre>](/application/zrc/images/qvar/CHEN11088716.png)
