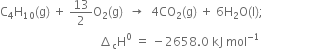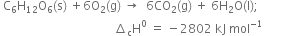What will be the amount of heat evolved when 39 gm of C6H6(l) are burnt? Given that:![]()
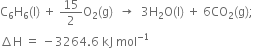



 would be =
would be = 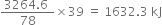
Given:![]()
What is the standard enthalpy of formation of NH3 gas?
The enthalpy of formation of ammonia is the enthalpy change for the formation of 1 mole of ammonia from its elements.
Given that enthalpy of formation for 2 moles of NH3 = –92.4 kJ
Therefore, standard enthalpy of formation for
Hence standard enthalpy of formation for NH3

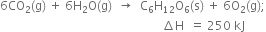

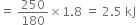
What do you mean by the standard enthalpy of combustion?