
Calculate the atomic mass(average) of chlorine using the following data:
| %Natural Abundance | Molar mass | |
| 35Cl | 75·77 | 34·9689 |
| 37Cl | 24·23 | 36·9659 |
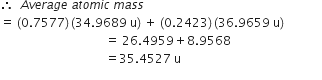
Calculate the amount of carbon dioxide that could be produced when
(i) 1 mole of carbon is burnt in air.
(ii) 1 mole of carbon is burnt in 16g of dioxygen.
(iii) 2 moles of carbon are burnt in 16g of dioxygen.
Calculate the mass percentage of different elements present in sodium sulphate (Na2SO4).
Molecular mass of sodium =23
Molecular mass of sulphur =32
Molecular mass of oxygen =16
Thus,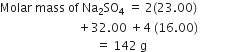
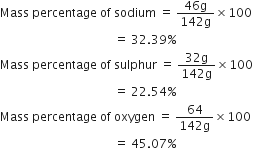
|
Element |
Percentage |
Atomic Mass |
Atomic ration |
Simplest ratio |
Simplest whole number ratio |
|
Iron (Fe) |
69.9 |
55.85 |
69.9/55.85=1.25 |
1.25/1.25 =1 |
2 |
|
Oxygen (O) |
30.1 |
16.00 |
30.1/16.00=1.88 |
1.88/1.25=1.5 |
3 |
