
Chlorination of methane is a free radical reaction which occurs by the following mechanism:
3
(a) Chain initiating step:
(b) Chain propagating step.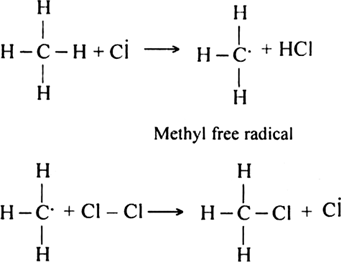
(c) Chain terminating step: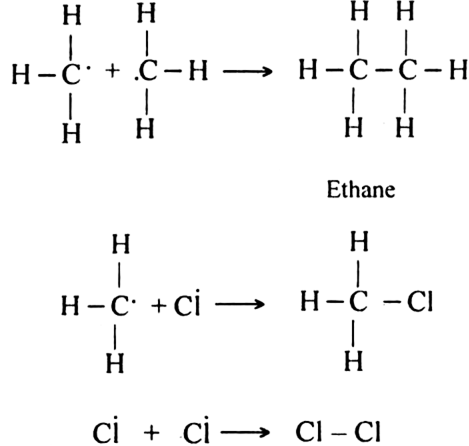
Since during the chain propagating step, CH3 free radicals are produced. This two methyl free radicals combine together in the chain-terminating step to form an ethane (CH3 - CH3) molecule.
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
1-Phenylbut-1-ene
![]()
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
3, 4-Dimethlhept-3-ene
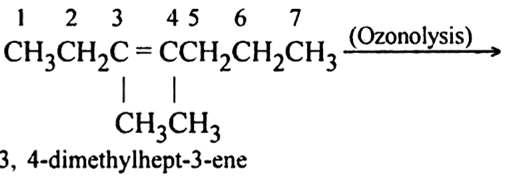
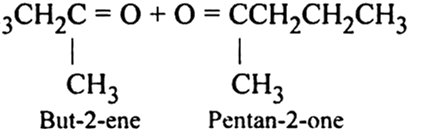
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
Pent - 2-ene

