Fill in the blanks with the choices given in brackets.
_____(AgCl/PbCl2), a white precipitate is soluble in excess NH4OH.
AgCl
By drawing an electron dot diagram show the formation of Ammonium ion.
[Atomic No.: N =7 and H = 1]
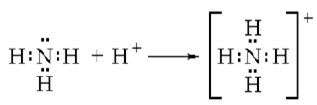
State your observations when ammonium hydroxide solution is added drop by drop and then in excess to each of the following solutions:
i) Copper sulphate solution
ii) Zinc sulphate solution.
i) When NH4OH is added to copper sulphate solution drop-wise, a pale blue ppt. is obtained.
CuSO4 +2NH4OH ---> Cu(OH)2 +(NH4)2SO4 +4H2O
With an excess of NH4OH, the ppt. dissolves to give a deep blue solution of tetraamine copper(II)sulphate.
Cu(OH)2 +(NH4)2SO4 +2NH4OH --> [Cu(NH3)4)SO4 +H2O
ii) When NH4OH is added to zinc sulphate solution drop-wise, a white, gelatinous ppt. is obtained.
ZnSO4 +2NH4OH --> Zn(OH)2 + (NH4)2SO4
With an excess of NH4OH, the ppt. dissolves to give a colourless solution of tetraamine zinc(II)sulphate.
Zn(OH)2 +(NH4)2SO4 +2NH4OH --> [Zn(NH3)4]SO4 +4H2O
Write balanced chemical equations for each of the following:
Action of warm water on AlN
AlN(s) + 3H2O(l) → Al(OH)3(aq) + NH3(g)
