Match the salts given in Column I with their method of preparation given in Column II:
| A. Pb(NO3)2 from PbO | (i) Simple displacement |
| B. MgCl2 from Mg | (ii) Titration |
| C. FeCl3 from Fe | (iii) Neutralization |
| D. FeCl3 from Fe | (iv) Precipitation |
| E. ZnCO3 from ZnSO4 | (v) Combination |
A. Pb(NO3)2 from PbO | (i) Precipitation |
B. MgCl2 from Mg | (ii) Simple displacement |
C. FeCl3 from Fe | (iii) Combination |
D. FeCl3 from Fe | (iv) Neutralization |
E. ZnCO3 from ZnSO4 | (v) Titration |
Choose the correct answer from the options given below:
The hydroxide of this metal is soluble is sodium hydroxide solution.
Magnesium
Lead
Silver
Silver
B.
Lead
Select the correct answer from the choices A, B, C and D which is given:
A black colour solid which on reaction with dilute sulphuric acid forms a blue coloured solution is:
Carbon
Manganese (IV) Oxide
Lead (II) Oxide
Lead (II) Oxide
D.
Lead (II) Oxide
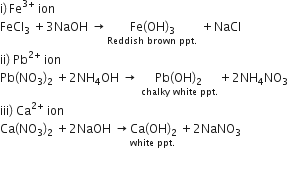
Sodium hydroxide solution is added to the solutions containing the ions mentioned in list X. List Y gives the details of the precipitate. Match the ions with their coloured precipitates.
| A. Pb2+ | (i) Reddish brown |
| B. Fe2+ | (ii) White insoluble in excess |
| C. Zn2+ | (iii) Dirty green |
| D. Zn2+ | (iv) White soluble in excess |
| E. Cu2+ | (v) White soluble in excess |
| F. Ca2+ | (vi) Blue |
A. Pb2+ | (i) White soluble in excess |
B. Fe2+ | (ii) Dirty green |
C. Zn2+ | (iii) White soluble in excess |
D. Zn2+ | (iv) Reddish brown |
E. Cu2+ | (v) Blue |
F. Ca2+ | (vi) White insoluble in excess |
Identify the cations in each of the following cases:
i) NaOH solution, when added to the solution (A), gives a reddish brown precipitate.
ii) NH4OH solution, when added to the solution (B), gives White ppt which does not dissolve is excess.
iii) NaOH solution, when added to Solution (C), gives white ppt which is insoluble in excess.