Choose the correct answer from the options given below:
The particles present in strong electrolytes are:
Only molecules
Mainly ions
Ions and molecules
Ions and molecules
B.
Mainly ions
Write equations for the reactions taking place at the two electrodes (mentioning clearly the name of the electrode) during the electrolysis of:
i) Acidified copper sulphate solution with copper electrodes.
ii) Molten lead bromide with inert electrodes.
i) 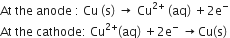
ii) The cathode and anode both are made of graphite plate:
At the anode: Br- --> Br + e-
At the cathode Pb2+ --> Pb +2e-
i) Name the product formed at the anode during the electrolysis of acidified water using platinum electrodes.
ii) Name the metallic ions that should be present in the electrolyte when an article made of copper is to be electroplated with silver.
i) When acidified water is electrolysis by using platinum electrode than oxygen gas is formed.
ii) The metallic ions are Ag+.
Give a reason why:
i) Sodium chloride will conduct electricity only in fused or aqueous solution state.
ii) in the electroplating of an article with silver, the electrolyte sodium argento-cyanide solution is preferred over silver nitrate solution.
iii) Although copper is a good conductor of electricity, it is a non-electrolyte.
(i)In the solid state, the electrostatic forces of attraction between the ions in the solid are very strong. Hence there are no free ions to conduct electricity.Although in the aqueous solution free ions present thus it conducts electricity.
(ii) Silver nitrate solution undergoes rapid dissociation that can cause non-uniform coating, therefore, it is not preferred whereas sodium argentocyanide to cyanide solution is a complex salt and undergoes slow decomposition and ensure smooth and uniform coating.
(iii) Copper has no mobile electrons in the solid state and an electrolyte should dissociate into oppositely charged ions to conduct electricity. Hence, copper is a non-electrolyte.
Fill in the blanks:
1) As we descend the electrochemical series containing cation, the tendency of the cation to get ............(oxidized/reduced) at the cathode increases.
2) The (highest/lower) ........... the concentration of an ion in a solution, the greater is the probability of its being discharged at its appropriate electrode.
reduce
,higher
