Osmotic Pressure. The pressure which is applied to the solution in order to prevent the passage of solvent into it through a semi-permeable membrane present between the two is termed as osmotic pressure.
We know that osmotic pressure is a colligative property and hence it depends on the number of solute particles.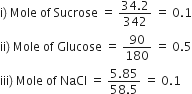
Since 1 mole = 6.023 x 1023 particles
So Sucrose = 6.023 x 1022 particles
(As Sucrose and Glucose are undissociated)
Glucose = 30.115 x 1022 particles
NaCl = 12.046 x 1022 particles.
(NaCl dissociates to give two ions)
Hence, the increasing order of their osmotic pressure is, Sucrose < NaCl < Glucose.
Match the following:
| A. Colligative property | (i) Polysaccharide |
| B. Nicol prism | (ii) Aldol condensation |
| C. Activation energy | (iii) Ammonia |
| D. Activation energy | (iv) Polarimeter |
| E. Acetaldehyde | (v) Arrhenius equation |
A. Colligative property | (i) Ammonia |
B. Nicol prism | (ii) Polarimeter |
C. Activation energy | (iii) Arrhenius equation |
D. Activation energy | (iv) Polysaccharide |
E. Acetaldehyde | (v) Aldol condensation |
| A. Elevation of boiling point | (i) Tetrahedral arrangement of atoms |
| B. Hydrogen fluride | (ii) Le-Chatelie’sprinciple |
| C. Diamond | (iii) Aluminium |
| D. Diamond | (iv) Relative lowering of vapour pressure |
| E. Cryolite | (v) Hydrogen bond |
A. Elevation of boiling point | (i) Relative lowering of vapour pressure |
B. Hydrogen fluride | (ii) Hydrogen bond |
C. Diamond | (iii) Tetrahedral arrangement of atoms |
D. Diamond | (iv) Le-Chatelie’sprinciple |
E. Cryolite | (v) Aluminium |
Complete the following statements by selecting the correct alternative from the choices given:
The molal freezing point constant of water is 1·86 K kg mol-1 . Therefore, the freezing point of 0·1M NaCl solution in water is expected to be:
-1·86oC
-0·372oC
-0·186oC
-0·186oC
B.
-0·372oC
Fill in the blanks by choosing the appropriate word/ words form those given in the brackets:
(Henry's, aldol condensation, absence, do not ohm, Raoult's, increases, common ion effect, easily, three, solubility product, ohm-1, two, four,ohm-1 cm2, cannizzaro, ohm-1 cm-1, zero decreases, presence.)
(i) Ideal solutions obey________ law and they_____ form azetropic mixtures.
(ii) Benzaldehyde undergoes_______ reaction due to _________ of alpha-hydrogen atom.
iii) The solubility of silver chloride ___________ in the presence of sodium chloride because of _______.
iv) The unit of conductance is ___________ and that of specific conductance is ________.
v) When the concentration of a reactant of first order reaction is doubled, the rate becomes _________ times, but for_________ order reaction, the rate remains same.
Raoult's , easily
,Cannizzaro, presence
,increase, common ion effect
,ohm-1, ohm-1 cm2
,four, zero
