
What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 at 30C?
From the given data,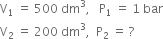
According to Boyle's law,

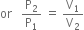
Substituting the values, we have,
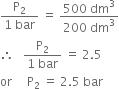
What would be the SI unit for the quantity ![]()
The unit of pressure is given as= Nm-2
The unit of volume is given as= m3
The unit of Temperature is given as= K2
Unit of mol is givne as = mol-1.
Thus SI unit of given quantity is given as,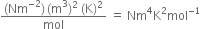
In terms of Charles’s law explain why –273°C is the lowest possible temperature.
The law states that at constant pressure, the volume of a fixed mass of gas is directly proportional to its absolute temperature. It was found that for all gases (at any given pressure), the plots of volume vs. temperature is a straight line. If this line is extended to zero volume, then it intersects the temperature axis at -2730C.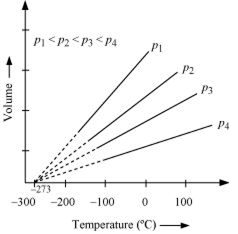
In other words, the volume of any gas at 273 0C is zero. This is because all gases get liquefied before reaching a temperature of 2730C.
Hence, it can be concluded that -273 0C is the lowest possible temperature
