For a linear plot of log (x/m) versus log p in a Freundlich adsorption isotherm, which of the following statements is correct? (k and n are constants)
1/n appears as the intercept.
Only 1/n appears as the slope.
log (1/n) appears as the intercept.
log (1/n) appears as the intercept.
B.
Only 1/n appears as the slope.
According to Freundlich adsorption isotherm,
On taking logarithm both sides, we get
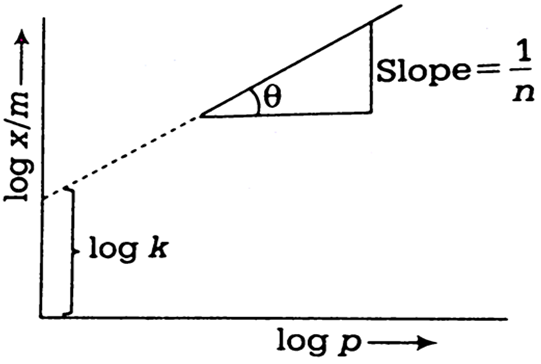
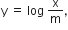 c= intercept = log k, m =slope = 1/n and x = log p
c= intercept = log k, m =slope = 1/n and x = log p
The Tyndall effect is observed only when following conditions are satisfied:-
(a) The diameter of the dispersed particles is much smaller than the wavelength of the light used.
(b) The diameter of the dispersed particle is not much smaller than the wavelength of the light used.
(c) The refractive indices of the dispersed phase and dispersion medium are almost similar in magnitude.
(d) The refractive indices of the dispersed phase and dispersion medium differ greatly in magnitude.
(a) and (d)
(b) and (d)
(a) and (c)
(a) and (c)
B.
(b) and (d)
The coagulating power of electrolytes having ions Na+, Al3+ and Ba2+ for arsenic sulphide sol increases in the order:
Al3+<Ba2+<Na+
Na+<Ba2+<Al3+
Ba2+< Na2+<Al3+
Ba2+< Na2+<Al3+
B.
Na+<Ba2+<Al3+
As2S3 is an anionic sol (negative sol) hence coagulation will depend upon coagulating power of cation, which is directly proportional to the valency of cation (Hardy-Schulze rule).
Which of the following statements is incorrect regarding physissorptions?
It occurs because of vander Waal’s forces.
More easily liquefiable gases are adsorbed readily.
Under high pressure, it results into multimolecular layer on the adsorbent surface.
Under high pressure, it results into multimolecular layer on the adsorbent surface.
D.
Under high pressure, it results into multimolecular layer on the adsorbent surface.
Enthalpy of adsorption in physisorption is negative
∆G = ∆H - T∆S
As the entropy decreases (∆S = –ve) the ∆H must be negative having a high magnitude. Therefore, the spontaneous adsorption will have negative enthalpy change.
