CsCl crystallises in body centred cubic lattice. If 'a' its edge length, then which of the following expression is correct?




C.

In CsCl, Cl- lie at corners of simple cube and Cs+ at the body centre, Hence, along the body diagonal, Cs+ and Cl- touch each other so
Sodium metal crystallises in a body centred cubic lattice with a unit cell edge of 4.29 Å The radius of sodium atom is approximate:
1.86 Å
3.22Å
5.72 Å
5.72 Å
A.
1.86 Å
According to the figure
(AC)2 = (AB)2 + (BC)2
(AC)2 = a2 + a2 = 2a2
(AD)2 = (AC)2+ (DC)2
(4r)2 = 2a2 + a2
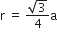
 =1.86Å
=1.86Å Lithium forms body centred cubic structure. The length of the side of its unit cell is 351 pm. Atomic radius of the lithium will be
75 pm
300 pm
240 pm
240 pm
D.
240 pm
In body centred cubic structure,
Edge length = a = 351 pm, radius= r=?
Which of the following compounds is metallic and ferromagnetic?
VO2
MnO2
TiO2
TiO2
D.
TiO2
Only three elements iron (Fe) cobalt (Co) and Nickel (Ni) show ferromagnetism at room temperature. CrO2 is also a metallic and ferromagnetic compound which is used to make magnetic tapes for cassette recorders
