What are the changes which take place when a redox reaction is carried in a beaker? Explain with the help of a suitable example.
Or
Explain the redox reaction
occurring in a beaker.
When a zinc rod is placed in an aqueous solution of copper sulphate, the following changes will be observed:
(i) The zinc plate loses weight gradually.
(ii) A precipitate of copper settles at the bottom of the beaker.
(iii) The blue colour of the solution gradually fades.
(iv) The solution remains electrically neutral throughout.
(v) The solution becomes hot (exothermic reaction).
The overall reaction which takes place in a beaker may be represented as:
Here, Zn is oxidised to Zn2+ ions by losing two electrons and Cu2+ ions are reduced to Cu(s).


As Cu2+ ions from the solution are changing to Cu(s), the blue colour of the solution which is due to Cu2+ ions, slowly fades. Also, the number of electrons lost in the oxidation half reaction is equal to the number of electrons gained in the reduction half reaction, the solution remains electrically neutral.
Similarly, when a copper rod is placed in a silver nitrate solution, silver gets precipitated with the evolution of heat energy. The reaction taking place in the beaker is:
Cancelling the common ion, 
Hence whenever a redox reaction is carried out in a single beaker, decrease in chemical energy or free energy appears in the form of heat.
Show that oxidation cannot occur without reduction.
Or
Show that oxidation and reduction go side by side.

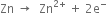

Define oxidising agents and reducing agents in terms of electronic concept ?
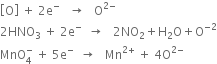
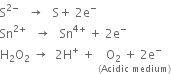

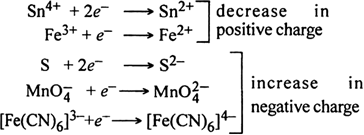

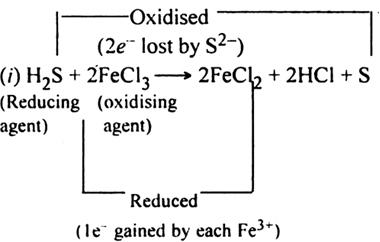
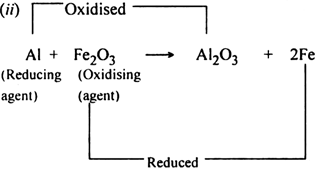
Here, Al reduces Fe2O3 to Fe while itself gets oxidised to Al2O3. Conversely, Fe2O3 oxidises Al to Al2O3 while itself gets reduced to Fe. Therefore Al acts as a reducing agent while Fe2O3 acts as an oxidising agent.
