Discuss the general trends in ionisation enthalpy and electropositive character of alkali metals.
Alkali metals possess lowest ionisation enthalpies in their respective periods. However, within the group, the ionisation enthalpies of alkali metals decrease down the group.
Reason: The atoms of alkali metals are largest in their respective periods, therefore, the outermost electrons, which are far away from the nucleus, experience a less force of attraction with the nucleus and hence can be easily removed. Decrease in ionisation enthalpy, on moving down the group, is due to the increase in the size of the atoms of alkali metals and increase in the magnitude of screening effect is by virtue of an increase in the number of intervening electrons.
Electropositive character: On account of their low ionisation enthalpies, these metals have a strong tendency to lose their valence electrons and thus change into positive ions. Consequently, alkali metals are strongly electropositive or metallic in character. As this tendency for losing electron increases down the group, the electropositive character increases.
Explain: Lithium exhibits anamalous behaviour in the company of alkali metals.
Or
Name the chief factor responsible for the anomalous behaviour of lithium.
Or
List three properties of lithium in which it differs from the rest of the alkali metals.
Anomalous behaviour of lithium is due to its:
(i) very small size,
(ii) high electronegativity and ionisation energy enthalpy value and
(iii) the absence of d-orbitals in the valence shell of its atom.
Therefore, lithium differs from other members of the family in the following respects:
(i) Lithium is harder than other alkali metals.
(ii) Lithium combines with oxygen to form lithium oxide while other alkali metals form peroxides and superoxides.
(iii) Lithium when heated with ammonia forms imide, Li2NH, while other alkali metals form amides, MNH2 as: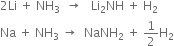
(iv) Lithium hydroxide and lithium carbonate decompose on heating while the hydroxides and carbonates of other alkali metals do not decompose on heating.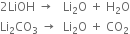
(v) Lithium unlike other alkali metals from no ethynide on reaction with ethyne.
(vi) Lithium nitrate when heated gives lithium oxide, while other alkali metal nitrates decompose to give the corresponding nitrites. 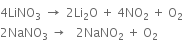
Lithium forms normal oxide, sodium forms peroxides while K, Rb and Cs form superoxides. Explain.
Lithium forms normal oxide  Lithium-ion with small size has a strong positive field around it. On combination with oxide anion, the positive field of lithium ion restricts the spread of negative charge towards another oxygen atom and thus prevents the formation of a higher oxide.
Lithium-ion with small size has a strong positive field around it. On combination with oxide anion, the positive field of lithium ion restricts the spread of negative charge towards another oxygen atom and thus prevents the formation of a higher oxide.
Sodium reacts with dioxygen to form sodium peroxide 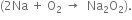 Sodium ion with a larger size than lithium ion has weaker positive field than lithium ion. This positive field is so weak that it cannot prevent the conversion of the oxide anion
Sodium ion with a larger size than lithium ion has weaker positive field than lithium ion. This positive field is so weak that it cannot prevent the conversion of the oxide anion 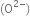 into a peroxide ion
into a peroxide ion 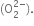 , However, it is strong enough to prevent further oxidation of peroxide to superoxide.
, However, it is strong enough to prevent further oxidation of peroxide to superoxide.
Potassium, rubidium and caesium react with dioxygen to form superoxide 
Potassium, rubidium and caesium ions are large sized and thus have a very weak positive field around them. The positive field around these ions is so weak that it cannot prevent the conversion of peroxide 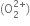 anion to superoxide anion
anion to superoxide anion 
Lithium is the strongest reducing agent in aqueous solution. Explain.
Electrode potential is the measure of the tendency of an element to lose electrons in the aqueous solution. Thus, more negative is the electrode potential, higher is the tendency of the element to lose electrons and hence stronger is the reducing agent.
Since the standard electrode potential  of
of
alkali metals become more and more negative as we move down the group from Na to Cs, therefore, reducing character of these elements increases in the same order i.e. Na to Cs. However, standard electrode potential (reduction) of lithium is the lowest i.e. -3.05 volts. In other words, lithium is the strongest reducing agent in the aqueous solution.
Reason. Electrode potential depends on :
(i) heat of sublimation
(ii) ionisation enthalpy
(iii) heat of hydration.
The sublimation enthalpies of alkali metals are almost similar. Now since Li+ ion is smallest in size, therefore, the large amount of energy released in step III (heat of hydration) compensates for the higher ionisation enthalpies, thereby facilitating the release of electron and hence explains the low value of electrode potential 
Name the elements of group 1. Write their electronic configurations.
The metallic elements lithium (Li), sodium (Na), potassium(K), rubidium (Rb), caesium (Cs) and francium (Fr) constitute group 1 of the periodic table. Francium is a radioactive element. They are known as alkali metals because their hydroxides are strong alkalies or bases.
Electronic configuration: The atoms of alkali metals have one electron in s-orbital outside a noble gas core. Therefore, their general electronic configuration is:
[Noble gas]ns1 where n = 2 to 7
The electronic configurations of alkali metals are given below:
| Element | At No | Electronic configuration |
| Lithium (Li) | 3 | [He]2s1 |
| Sodium (Na) | 11 | [Ne] 3s1 |
| Potassium (K) | 19 | [Ar] 4s1 |
| Rubidium (Rb) | 37 | [Kr] 5s1 |
| Caesium (Cs) | 55 | [Xe] 6s1 |
| Francium (Fr) | 87 |
[Rb] 7s1 |
