
a)
b) A is better for coating the surface of the iron because its E0 value is more negative.
Or
a) 
b) A device used for the production of electricity from energy released during the spontaneous chemical reaction and the use of electrical energy to bring about a chemical change. The reaction gets reversed / It starts acting as an electrolytic cell & vice – verse.
Galvanization is applying a coating of:
Cr
Cu
Zn
Zn
C.
Zn
Zinc metal is the most stable metal to cover iron surfaces. The process of coating the iron surface by zinc is called galvanization.
(a) What type of a battery is lead storage battery? Write the anode and cathode reactions and the overall cell reaction occurring in the operation of a lead storage battery.
(b) Calculate the potential for half-cell containing 0.10 M K2Cr2O7 (aq), 0.20 M Cr3+(aq) and 1.0 x 10-4 M H+ (aq)
The half-cell reaction is 
And the standard electrode potential is given as E0 = 1.33 V.
OR
(a) How many moles of mercury will be produced by electrolysing 1.0 M?
Hg (NO3)2 solution with a current of 2.00 A for 3 hours?
[Hg (NO3)2 = 200.6 g mol-1]
(b) A voltaic cell is set up at 25°C with the following half-cells Al3+ (0.001 M) and Ni2+ (0.50 M). Write an equation for the reaction that occurs when the cell generates an electric current and determine the cell potential.

(a) A lead storage battery is a secondary battery.
The following chemical equations take place in a lead storage battery.
: 
When a battery is charged, the reverse of all these reactions takes place.
Hence, on charging, PbSO4(s) present at the anode and cathode is converted into Pb(s) and PbO2(s) respectively.
b) 
Or
(a) Quantity of electricity passed = (2A) x (3 x 60 x 60s) = 21600 C
Thus, 2F i.e. 2 x 96500 C deposit Hg = 1 mole 21600 C will deposit Hg
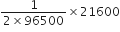
= 0.11 mole
Or
At anode: Al (s) --> Al3+ (aq) + 3e-] x2
At cathode: Ni2+ + 2e- --> Ni(s) ] x3
Cell reaction: 2Al(s) + 3Ni2+(aq) ---> 2Al3+(aq) + 3Ni (s)
Applying nernst equation to the above cell reaction 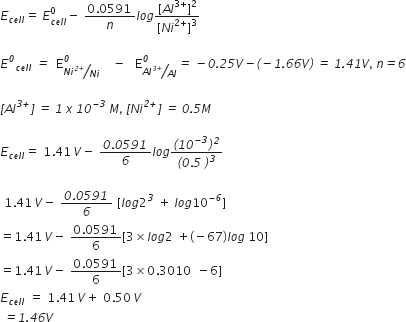
Two Faraday of electricity is passed through a solution of CuSO4. The mass of copper deposited at the cathode is: (at. mass of Cu = 63.5 amu)
0 g
63.5 g
2 g
2 g
B.
63.5 g
Atomic mass of Cu = 63.5 u
Valency of the metal Z= 2
We have,
CuSO4 → Cu2+ + SO42-
Cu2+ + 2e- → Cu
