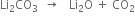When an alkali metal dissolves in liquid ammonia the solution can acquire different colours. Explain the reactions for this type of colour change.
Or
Explain why alkali metals dissolve in liquid ammonia to form deep blue solution.
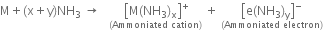
Find out the oxidation state of sodium in Na2O2.
Oxidation state of Oxygen Na2O2 is =(-1)
Let x be the oxidation state of Na in Na2O2. Since peroxide linkage is present in Na2O2 in which the oxidation of O is –1.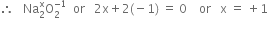
Hence oxidation state of Na in 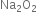 is +1.
is +1.
Why are alkali metals not found in nature?
Alkali metal has one electron each in the valence subshell of their atoms. Since they have only one electron in valence subshell, therefore, they lose easily, owing to their low ionisation energies. Therefore, alkali metals are highly reactive chemically and do not exist in the free or native state.
Why is Li2CO3 decomposed at a lower temperature while Na2CO3 at higher temperature?