
Calculate the atomic mass(average) of chlorine using the following data:
| %Natural Abundance | Molar mass | |
| 35Cl | 75·77 | 34·9689 |
| 37Cl | 24·23 | 36·9659 |
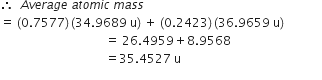
Calculate the amount of carbon dioxide that could be produced when
(i) 1 mole of carbon is burnt in air.
(ii) 1 mole of carbon is burnt in 16g of dioxygen.
(iii) 2 moles of carbon are burnt in 16g of dioxygen.
Calculate the mass of sodium acetate (CH3COONa) required to make 500 mL of 0·375 molar aqueous solution. Molar mass of sodium acetate is 82·0245g mol–1.
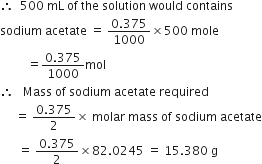
Calculate the mass percentage of different elements present in sodium sulphate (Na2SO4).
Molecular mass of sodium =23
Molecular mass of sulphur =32
Molecular mass of oxygen =16
Thus,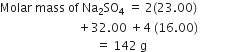
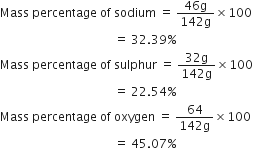
|
Element |
Percentage |
Atomic Mass |
Atomic ration |
Simplest ratio |
Simplest whole number ratio |
|
Iron (Fe) |
69.9 |
55.85 |
69.9/55.85=1.25 |
1.25/1.25 =1 |
2 |
|
Oxygen (O) |
30.1 |
16.00 |
30.1/16.00=1.88 |
1.88/1.25=1.5 |
3 |
