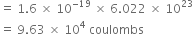(a) 1 g atom of carbon = 14g
= 6.022 x 1023 atoms
Each carbon atom 

(b) Mass of 1 neutron = 1.675 x 10-27 kg![]() Mass of 2.4088 x 1021 neutrons
Mass of 2.4088 x 1021 neutrons
= (1.675 x 10-27kg) x (2.4088 x 1021)
= 4.0347 x 10-6kg
Write the complete symbol for the atom with the given atomic number (Z) and atomic number (A).
(i) Z = 17, A = 35
(ii) Z = 92, A = 233
(iii) Z = 4, A = 9.
(i) The element with atomic number 17 is Cl. Its symbol is ![]()
(ii) The element with atomic number 92 is U. Its symbol is ![]()
(iii) The element with atomic number 4 is Be. Its symbol is ![]()
Calculate the number of electrons which together weigh one gram.
Mass of one electron =9.10939 x 10-31 kg
Therefore, Number of electrons that weigh 9.10939 x 10-31 kg =1
Number of electrons that will weigh1g= (1x10-3kg)
=0.1098 X10-3+31
=0.1098 X1028
=1.098 X1027
Calculate the mass and charge of one mole of electrons.
One mole of electron
![]()
Mass of 1 electron = ![]()
Mass of ![]()
![]()
Charge on one electron
![]()
Charge on one mole electrons