 Short Answer Type
Short Answer TypeCalculate the percentage of sodium in sodium aluminium fluoride (Na3AlF6) correct to the nearest whole number. (F=19;Na=23; Al=27).
Molecular weight of Na2AlF6 = 3 x 23 +27+19 x 6
В В В В В В В В В В В В В В В В В В В В В = 69+27+114В
В В В В В В В В В В В В В В В В В В В В В = 210
Weight of sodium В = 3 x 23 =69
% of sodium =В 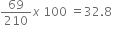
В 560mklВ of carbon monoxide is mixed with 500ml of oxygen and ignited. The chemical equation for the reaction is as follows:
2CO2 +O2 ---> 2CO2
Calculate the volume of oxygen used and carbon dioxide formed in the above reaction.
Select from the list given below (A to F), the one substance in each case which matches the descriptions given in parts (ii) &(iii). Copy and complete the given grid with your answer as shown for part(i)
|
(i) |
(ii) |
(iii) |
|
A |
В |
В |
В (A)В В Ammonia
(B)В В Copper oxide
(C)В В Copper sulphate
(D)В В Hydrogen chloride
(E)В В В Hydrogen sulphide
(F)В В В Lead bromide
(i) Although this compound is not a metal hydroxide. Its aqueous solution is alkaline in nature.
(ii) A solution of this compound is used as the electrolyte when copper is purified
(iii) When this compound is electrolysed in the molten state, lead is obtained atВ the cathode.
State what is observed when:
Copper sulphate solution is electrolyzed using a platinum anode.
study the diagram given below and answer the questions thatВ follows: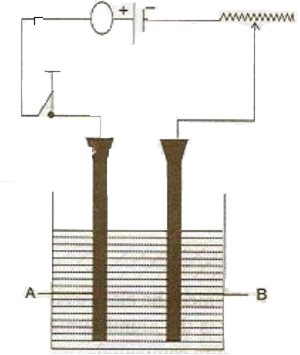
i)В
(1)В В В Give the name of the electrode A and B.
(2)В В В Which electrode is the oxidising electrode?
iii) write the equation of the reactions which take place atВ the cathode and anode when acidified water is electrolyzed.
Give reason for the following:
A solution of silver nitrate is a good electrolyte but it is not used for electroplating an article with silver
Q1. (a) select from the list given below (A toF) the one substance in each case which matchesВ the descriptions given in part (vi). Copy and complete the given grid with your answers as shownВ for part (i).
|
(i) |
(vi) |
|
A |
В |
A. Ammonia
B. Copper oxide
C. Copper sulphate
D. hydrogen Chloride
E. Hydrogen sulphide'
F. Lead bromide'
i) although this compound is not a metal hydroxide, its aqueous В solution is alkaline in nature.
iv) This compound can be reduced to copper when heated with coke.
Write a balanced chemical equation for the following reaction:
Aluminium oxide and sodium hydroxide solution
Write balanced chemical equation for the following reaction:
Carbon and carbon dioxide.
