 Short Answer Type
Short Answer TypeGiven, Molarity = 0.05 M, R = 31.6 ohms
cell constant = 0.378 cm-1, Molar conductivity (∧m) = ?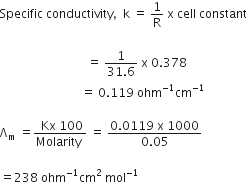
Give a reason for each of the following :
The number of hydrogen ions in an aqueous solution of acetic acid increase considerably with dilution while this is not the case with an aqueous solution of hydrogen chloride.
Give a reason for each of the following :
A mixture of NH4Cl and NH4OH is used to precipitate the metallic hydroxides of group III.
Arrange the following in increasing order of acidity and explain your order:
Phenol, Methanol, Water.
