 Multiple Choice Questions
Multiple Choice QuestionsThe order of first ionization energies of the elements Li, Be, B, Na is :
Li> Be> B > Na
Be > B > Li > Na
Na > Li > B> Be
Be > Li > B > Na
B.
Be > B > Li > Na
Ionization energies increase in a period on moving left to right while it decreases in a group on moving downward. The I.E. of Be is greater than B due to completely filled s-orbital. Hence, the order of IE is as :
Be> B >Li> Na
Calculate the mass loss in the following.
[Given the masses : 2H = 2.014;3H = 3.016; He = 4.004; n = 1.008 amu]
0.018 amu
0.18 amu
0.0018 amu
1.8 amu
Δ H and ΔS for a reaction are +30.558 kJ mol-1 and 0.066 kJ K-1mol-1 at 1 atm pressure. The temperature at which free energy change is equal to zero and the nature of the reaction below this temperature are :
483 K, spontaneous
443 K, non-spontaneous
443 K, spontaneous
463 K, non-spontaneous
An alkene having the molecular formula C9H18 on ozonolysis gives 2, 2-dimethyl propanal and 2-butanone. The alkene is :
2, 2, 2-trimethyl-3-hexene
2, 2, 6-trimethyl-3-hexane
2, 3, 4-trimethyl-2-hexene
2, 2, 4-trimethyl-3-hexene
Observe the following reactions and predict the nature of A and B:
A and B both are 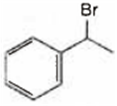
A and B both are ![]()
A is 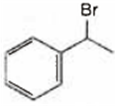 and B is
and B is ![]()
A is ![]() and B is
and B is 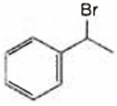
Nitration of aniline in strongly acidic medium, result in the formation of m-nitroaniline also. This is because :
amino group is meta orienting during electrophilic substitution reaction
nitro group goes always to the meta position irrespective of the substituents
nitration of aniline is a nucleophilic substitution reaction in strongly acidic medium
in strongly acidic conditions aniline is present as anilinium ion
The name of the compound fig is:
![]()
(2Z, 4Z)-2, 4-hexadiene
(2Z, 4E)-2, 4-hexadiene
(2E, 4Z)-2, 4-hexadiene
( 4E, 4Z)-2, 4-hexadiene
When 32.25 g of ethyl chloride is subjected to dehydrohalogenation reaction the yield of the alkene formed is 50%. The mass of the product formed is (atomic mass of chlorine is 35.5).
14 g
28 g
64.5 g
7 g
