 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following compounds is not chiral?
1-chloro-2-methyl pentane
2-chloropentane
1-chloropentane
3-chloro-2-methyl pentane
C.
1-chloropentane
To be optically active, compound or structure should possess a chiral or asymmetric carbon atom.
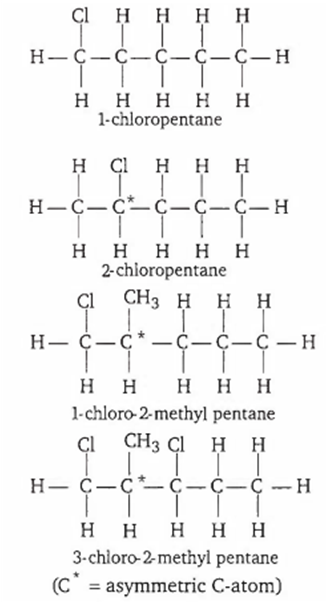
Hence, 1-chloropentane is not chiral.
The point of dissimilarity between lanthanides and actinides is
three outermost shells are partially filled
they show oxidation state of + 3 (common)
they are called inner transition elements
they are radioactive in nature
The general electronic configuration of the transition elements is
(n-1)d10, (n+1)s2
(n- 1)d1-10, (n + 1)s1-2
(n- 1)d1-10, np6,ns2
(n- 1)d1-10, ns1-2
Solutions A, B, C and D are respectively 0.1 M glucose, 0.05 M NaCl, 0.05 M BaCl2 and 0.1 M AlCl3. Which one of the following pairs is isotonic?
A and B
B and C
A and D
A and C
The vapour pressure of benzene at a certain temperature is 640 mm of Hg. A non-volatile and non-electrolyte solid weighing 2.175 g is added to 39.08 g of benzene. If the vapour pressure of the solution is 600 mm of Hg, what is the molecular weight of solid substance ?
49.50
59.60
69.60
79.82
