 Multiple Choice Questions
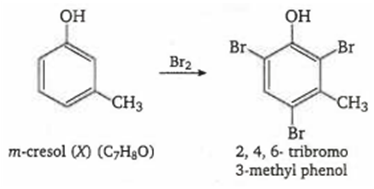
Multiple Choice QuestionsAn organic compound 'X with molecular formula, C7H8O is insoluble in aqueous NaHCO3, but dissolves in NaOH. When treated with bromine water 'X' rapidly gives 'Y, C7H5OBr3. The compounds 'X' and 'Y' respectively, are
benzyl alcohol and 2,4,6-tribromo-3-methoxy benzene
m-cresol and 2,4,6-tribromo-3-methyl phenol
benzyl alcohol and 2,4,6-tribromo-3-methyl phenol
o-cresol and 3,4,5-tribromo-2-methyl phenol
B.
m-cresol and 2,4,6-tribromo-3-methyl phenol
Compound' X' (C7H8O) is insoluble in aqueous NaHCO3 but soluble in NaOH, so it is a phenol. Since the number of carbon atoms remains the same after bromination, the compound must be meta cresol and reactions takes place as follows :
The products obtained when benzyl phenyl ether is heated with HI in the mole ratio 1 : 1 are
1.phenol
2. benzyl alcohol
3. benzyl iodide
4. iodobenzene
1 and 3 only
3 and 4 only
1 and 4 only
2 and 4 only
The correct order of increasing basic nature of the following bases is
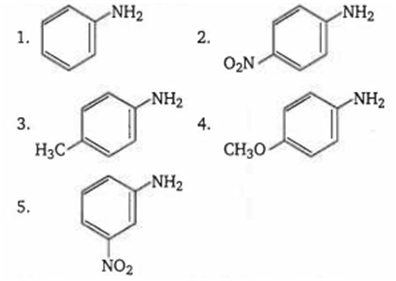
2 < 5 < 1 < 3 < 4
5 < 2 < 1 < 3 < 4
2 < 5 < 1 < 4 < 3
5 < 2 < 1 < 4 < 3
Which one of the following is a non-steroidal hormone?
Estradiol
Prostaglandin
Progesterone
Estrone
Which set of term correctly identifies the carbohydrate show?

1.Pentose
2. Hexose
3. Aldose
4. Ketose
5. Pyranose
6. Furanose
1, 3 and 6
1, 3, and 5
2, 3 and 5
2, 3 and 6
Which one of the following compounds is capable of existing in a mesa form?
2,4-dibromopentane
3,3-dibromopentane
4-bromo-2-pentanol
3-bromo-2-pentanol
