Multiple Choice Questions
Multiple Choice QuestionsAn organic amino compound reacts with aqueous nitrous acid at low temperature to produce an oily nitroso amine. The compound is
CH3NH2
CH3CH2NH2
CH3CH2NHCH2CH3
(CH3CH2)3N
C.
CH3CH2NHCH2CH3
Organic compound + HNO2 oily nitrosoamine
Hence, the organic compound is a secondary amine and therefore, the compound is CH3CH2NHCH2CH3 .
The property which distinguishes formic acid from acetic acid is
only ammonium salt of formic acid on heating gives amide
when heated with alcohol/ H2SO4 only acetic acid forms ester
only acetic acid forms salts with alkali
only formic acid reduces Fehling's solution
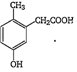
P-cresol reacts with chloroform in alkaline medium to give the compound A which adds hydrogen cyanide to form the compound B. The latter on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is