 Multiple Choice Questions
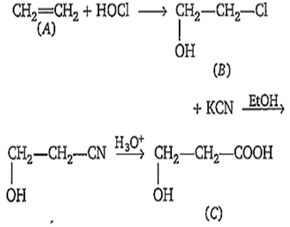
Multiple Choice QuestionsOne percent composition of an organic compound A is, carbon : 85.71% and hydrogen 14.29%. Its vapour density is 14. Consider the following reaction sequence-
Identify .
CH3CH(OH)-CO2H
HO-CH2-CH2-CO2H
HO-CH2-CO2H
CH3-CH2-CO2H
B.
HO-CH2-CH2-CO2H
C = 85.71% = = 7.14; = 1
H = 14.29% = = 14.29; = 2
Empirical formula = CH2
and empirical formula weight = 12 + 2 = 14;
Again,
Molecular formula weight = 2 × vapour density
= 2 × 14 = 28
n = = 2
Molecular formula = (CH2)2 = C2H4
At 25°C, the molar conductances at infinite dilution for the strong electrolytes NaOH, NaCl and BaCl2 are 248 × 10-4, 126 × 10-4 and 280 × 10-4 Sm2 mol-1 respectively, (BaOH)2 in Sm2 mol-1 is
52.4 × 10-4
524 × 10-4
402 × 10-4
262 × 10-4
The equilibnum constant for the given reaction is 100.
N2 (g) + 2O2 (g) 2NO2 (g)
What is the equilibrium constant for the reaction given below?
NO2 (g) (g) + O2 (g)
10
1
0.1
0.01
20 mL of 0.1 M acetic acid is mixed with 50 mL of potassium acetate. Ka of acetic acid = 1.8 × 10-5 at 27°C. Calculate concentration of potassium acetate if pH of the mixture is 4.8.
0.1 M
0.04 M
0.4 M
0.02 M
Calculate for the reaction,
Na2O (s) + SO3 (g) → Na2SO4 (g) given the following-
(A) Na (s) + H2O (l) → NaOH (s) + H2 (g) ; H° = -146 kJ
(B) Na2SO4 (s) + H2O (l) → 2NaOH (s) + SO3 (g) ; = +418 kJ
(C) 2Na2O (s) + 2H2 (g) → 4Na (s) + 2H2O (l); = +259 kJ
+823 kJ
-581 kJ
-435 kJ
+531 kJ
One mole of alkene on ozonolysis gave one mole of acetaldehyde and one mole of acetone. The IUPAC name of is
2-methyl-2-butene
2-methyl-1-butene
2-butene
1-butene
The chemical entities present in thermosphere of the atmosphere are
O, O+, NO+
O3
N2, O2, CO2, H2O
O3, O, O2
SiCl4 on hydrolysis forms 'X' and HCl. Compound 'X' loses water at 1000°C and gives 'Y'. Compounds 'X' and 'Y' respectively are
H2SiCl6, SiO2
H4SiO4, Si
SiO2, Si
H4SiO4, SiO2
Assertion (A) - K, Rb and Cs form superoxides.
Reason (R) - The stability of the superoxides increases from K to Cs due to decrease in lattice energy.
The correct answer is
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true but (R) is not true.
(A) is not true but (R) is true.
How many mL of perhydrol is required to produce sufficient oxygen which can be used to completely convert 2 L of SO2 gas to SO3 gas?
10 mL
5 mL
20 mL
30 mL
